Medtronic (NYSE: MDT) has taken a major step forward in its robotic surgery program, announcing the launch of Embrace Gynecology, a U.S. FDA investigational device exemption (IDE) study evaluating its Hugo robotic-assisted surgery platform for gynecological procedures. The clinical trial marks Medtronic’s third IDE study for Hugo in the U.S. and signals the company’s expanding push into the surgical robotics market currently dominated by Intuitive Surgical.
The first successful procedures under the study — robotic-assisted total hysterectomies — were performed by Dr. Sarah Crafton and Dr. Eirwen Miller at AHN West Penn Hospital in Pittsburgh, Pennsylvania. These initial cases represent the start of a broader investigation into Hugo’s use for radical, modified radical, and total hysterectomies, including cases involving malignancies. Medtronic plans to enroll up to 70 patients across five U.S. hospitals, evaluating both the safety and effectiveness of the system in a gynecologic setting.
“This is an exciting moment for both clinicians and patients,” said Dr. Emma Rossi, national principal investigator for the Embrace Gynecology study and Associate Professor of Obstetrics and Gynecology at Duke University. “In my experience, women facing a gynecologic diagnosis want two things: to effectively treat their condition and to get back to their full lives as quickly as possible. Robotic-assisted surgery helps make that possible.”
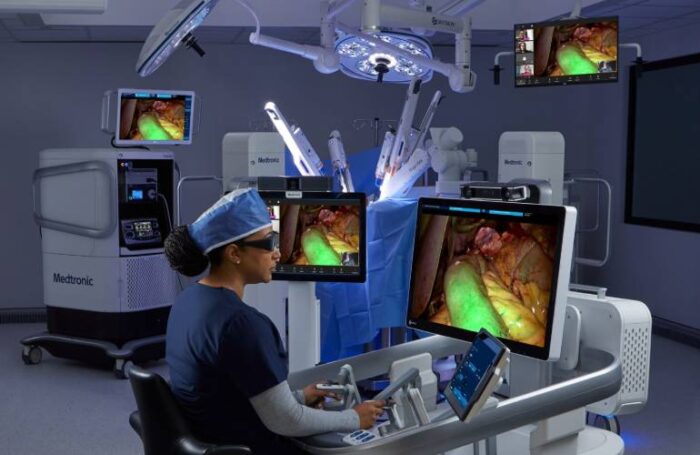
The Hugo robotic-assisted surgery (RAS) system is a modular, multi-quadrant platform built to provide flexibility across various surgical specialties. It features wristed instruments for precision movement, 3D high-definition visualization, and Touch Surgery Enterprise integration — Medtronic’s cloud-based digital ecosystem for surgical video capture and analytics. Together, these tools create a highly connected operating room experience designed to support surgical teams with real-time data and performance insights.
Medtronic first unveiled Hugo in September 2019, positioning it as a scalable and cost-efficient alternative to Intuitive’s da Vinci system, which has led the surgical robotics field for over two decades. The company describes Hugo as a “next-generation robotic platform” that aims to democratize access to robotic-assisted surgery by reducing barriers related to cost, footprint, and system complexity — key considerations for hospitals looking to expand their robotic capabilities.
The Embrace Gynecology study represents a crucial step toward securing FDA clearance for Hugo’s gynecology indication. If successful, it could further expand Hugo’s presence in the U.S., where Medtronic is currently pursuing multiple indications. The company said the new study follows previous positive results in its urology (Expand URO) and hernia repair IDE studies, both of which met their primary safety and efficacy endpoints earlier this year.
According to Medtronic, robotic-assisted surgery in gynecology offers significant benefits over traditional open approaches — including fewer complications, shorter hospital stays, and faster recovery times. With Hugo’s precision instrumentation and advanced imaging, surgeons can operate with enhanced dexterity and visualization, particularly in complex procedures involving delicate pelvic anatomy.
“The study name, Embrace, reflects our deeply felt compassion and care for patients, and our commitment to providing access to less invasive treatment options for women,” said Dr. James Porter, Chief Medical Officer of Robotic Surgical Technologies and Digital Technologies within Medtronic’s Surgical business. “We are grateful for the strong partnership with clinical teams at our study sites and share their excitement about this rigorous scientific study that is helping to usher in a new era of choice for patients in the U.S.”
The initiation of this study further solidifies Medtronic’s strategic commitment to making robotic-assisted surgery more accessible and adaptable across multiple specialties, as it prepares for an anticipated U.S. market entry in late fiscal year 2025.
The launch of the Embrace Gynecology IDE study marks a pivotal milestone for Medtronic’s Hugo robotic-assisted surgery (RAS) platform, signaling the company’s growing determination to challenge the dominance of Intuitive Surgical’s da Vinci system and redefine the competitive landscape of robotic surgery. With the Hugo system now entering its third U.S. IDE trial, Medtronic is steadily building the clinical foundation required for FDA clearance and eventual market introduction in the United States.
A strategic expansion into women’s health
Medtronic’s decision to pursue a gynecology indication highlights a significant and fast-growing segment of the surgical robotics market. Hysterectomies and other gynecologic surgeries represent one of the largest categories of minimally invasive procedures globally, with millions performed annually. Yet, access to robotic-assisted surgery remains uneven due to cost barriers and limited hospital adoption.
By initiating the Embrace Gynecology study, Medtronic aims to demonstrate that Hugo can deliver comparable or superior clinical outcomes to traditional robotic systems, while offering hospitals a more scalable and economically flexible platform. This approach could help expand robotic adoption in both community hospitals and large academic medical centers — environments where cost and operational efficiency are key decision factors.
“Robotic-assisted surgery in gynecology continues to evolve as a cornerstone of minimally invasive care,” said Dr. Emma Rossi, national principal investigator for the study. “If we can show that Hugo achieves consistent, safe outcomes across different patient populations, it could open the door for broader access and a higher standard of care for women across the U.S.”
Building momentum through clinical validation
The Embrace study follows Hugo’s previous U.S. trials in urology (Expand URO) and hernia repair, both of which met their safety and efficacy endpoints. Those results have given Medtronic strong momentum as it works to secure regulatory approval. The company submitted Hugo’s soft tissue robotics platform to the FDA for a urology indication in early 2025, with an eye toward launching commercially in the second half of its fiscal year, ending April 2026.
If approved, Hugo would represent one of the most significant new entrants into the U.S. robotic surgery market in nearly two decades. Medtronic expects its modular system design, flexible instrument configurations, and Touch Surgery Enterprise connectivity to set it apart from established competitors.
Hugo’s technological edge
Medtronic’s Hugo system integrates several key advancements aimed at addressing pain points that surgeons and hospitals have long faced with robotic systems:
Modular, multi-quadrant architecture: Allows hospitals to scale the system based on procedure type and case volume.
Wristed instruments: Provide natural, human-like motion with greater dexterity and control.
3D visualization: Offers surgeons enhanced depth perception and detail during complex tissue dissection.
Digital video capture via Touch Surgery Enterprise: Enables seamless recording, sharing, and analytics of surgical cases for training and quality improvement.
These features, combined with a smaller footprint and lower acquisition cost, are designed to make Hugo more accessible for a broader range of healthcare institutions. In essence, Medtronic aims to deliver the clinical sophistication of high-end robotics with the practical flexibility of modular design — a combination it hopes will accelerate the democratization of robotic-assisted surgery.
Redefining Medtronic’s robotics roadmap
For Medtronic, the Embrace Gynecology study is not just about expanding Hugo’s indications — it’s about solidifying a long-term presence in the global robotic surgery market, estimated at over $18 billion by 2030. The company views Hugo as a cornerstone of its broader digital surgery ecosystem, which merges hardware, data, and analytics into a connected network of surgical intelligence.
Dr. James Porter, Chief Medical Officer of Robotic Surgical Technologies and Digital Technologies at Medtronic, emphasized the company’s patient-centered mission: “The study name, Embrace, reflects our compassion for patients and our commitment to giving women access to less invasive treatment options. This trial embodies our belief that robotic surgery can and should be accessible to all.”
A new phase in Medtronic’s U.S. robotic ambitions
Globally, Hugo has already been cleared for clinical use in several regions, including Europe, Latin America, and parts of Asia, where it has been deployed in a range of soft-tissue procedures. The U.S. trials are the final hurdle before the system can compete head-to-head with Intuitive Surgical and newer entrants like Johnson & Johnson’s Ottava system, still in development.
Analysts view Medtronic’s steady progress as a sign that the U.S. robotic surgery market — long dominated by a single player — is on the verge of diversification. With Hugo’s IDE studies advancing successfully, Medtronic is positioning itself to become a credible, full-scale competitor offering hospitals a viable alternative in price, design, and digital integration.
As the Embrace Gynecology trial moves forward, the company expects to gather comprehensive data demonstrating the platform’s safety, reproducibility, and procedural efficiency. Pending successful outcomes and FDA approval, Hugo could begin its U.S. commercial rollout as early as late fiscal 2025, ushering in a new era of competition and innovation in robotic-assisted surgery.
Powered by Froala Editor