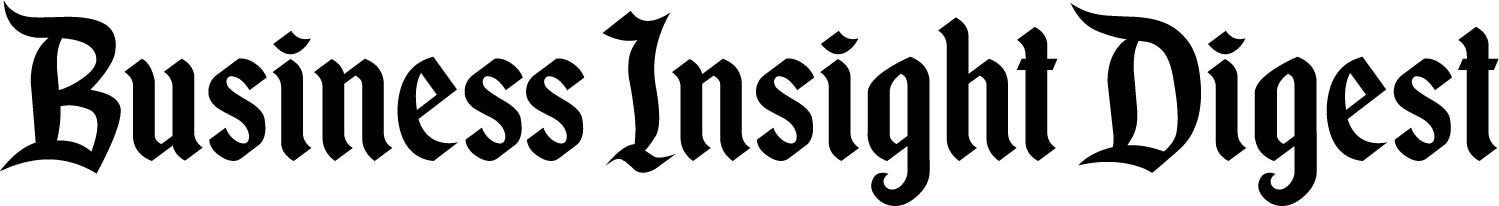The innovative MARS (magnetic-assisted robotic surgery) system from Levita Magnetics harnesses magnetic forces to precisely control surgical instruments inside patients, representing a significant advancement in minimally invasive surgery.
The company recently announced an expanded FDA 510(k) clearance for its MARS platform, combined with their Magnetic Surgical System (MSS), specifically for bariatric procedures and hiatal hernia repairs.
Expanded FDA Clearance
This expanded indication strengthens the company's ability to support surgeons treating obesity-related conditions by providing a less invasive solution that enables simultaneous treatment of hiatal hernias during bariatric surgery.
The new FDA clearance enables clinicians to leverage the system's Dynamic Magnetic Positioning technology, which facilitates liver retraction while enhancing surgical visibility and access during hiatal hernia repairs.
"This achievement reinforces our dedication to expanding access to superior surgical outcomes. We're shaping a future where less invasive approaches yield more effective results."
Innovative Magnetic Grasper
The FDA has also approved the company's new 12.5 mm magnetic grasper, specifically engineered to enhance visualization and control in patients with high BMI and challenging liver retraction cases.
This innovative instrument allows surgeons to grasp the liver more centrally rather than from the edge, significantly improving surgical access in both hernia and bariatric procedures.
MARS Technology Platform
MARS introduces a paradigm shift in robotic surgery by utilizing magnetic technology to reposition organs internally. This groundbreaking approach reduces the number of required incisions while enabling complete control during laparoscopic procedures.
Key Features
- Advanced magnetic positioning technology
- Reduced incision requirements
- Enhanced visualization
- Improved surgical access
- Seamless OR integration
Clinical Benefits
- Simultaneous condition treatment
- Enhanced surgical efficiency
- Improved patient outcomes
- Reduced procedural complexity
- Better visualization
Clinical Applications & Benefits
Bariatric Procedures
The expanded FDA clearance particularly benefits surgeons performing bariatric procedures, enabling simultaneous treatment of multiple conditions with enhanced efficiency and improved patient outcomes.
Hiatal Hernia Repairs
The system's ability to provide complete control during laparoscopic procedures while reducing invasiveness marks a significant advancement in surgical technology for complex abdominal procedures.
Future Development & Market Impact
The MARS platform continues to evolve, with ongoing development focused on expanding its applications across various surgical specialties. This latest FDA clearance represents a crucial step forward in the platform's growth, validating its effectiveness and safety in addressing complex surgical challenges.
As surgical robotics continue to advance, technologies like MARS demonstrate the potential for magnetic-assisted approaches to revolutionize minimally invasive surgery, offering surgeons enhanced control while prioritizing patient outcomes.
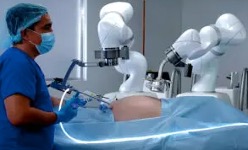
The Levita Magnetics MARS (magnetic-assisted robotic surgery) system uses magnetic forces to control a grasper instrument inside a patient. [Image courtesy of Levita Magnetics]