Boston Scientific (NYSE: BSX) has unveiled new data demonstrating the strength of its Farapulse pulsed field ablation (PFA) system and Watchman FLX left atrial appendage closure (LAAC) device, reinforcing its leadership in atrial fibrillation (AFib) therapies. The company presented the findings at the 2025 European Society of Cardiology (ESC) Congress in Madrid, one of the world’s premier stages for cardiovascular innovation.
Both devices target major unmet needs in AFib management: Farapulse provides a minimally invasive, nonthermal energy option for ablation, while Watchman FLX offers a solution for patients who require stroke protection but are unsuitable for long-term oral anticoagulation therapy. Together, they represent Boston Scientific’s dual-pronged strategy to address both rhythm control and stroke prevention in AFib care.
Farapulse: one-year data from FARADISE registry
The Farapulse PFA system has been at the center of Boston Scientific’s growth strategy since its $1.15 billion acquisition of Farapulse in 2021. The system received FDA approval in early 2024, followed by expanded approval in summer 2024, cementing its role as a next-generation alternative to radiofrequency (RF) and cryoablation.
At ESC 2025, Boston Scientific shared one-year outcomes from the FARADISE global registry, which enrolled more than 1,100 patients across a variety of ablation strategies and AFib types, including both paroxysmal and persistent AFib.
Key findings included:
Favorable procedural safety: Farapulse demonstrated a 1.5% serious adverse event rate, a low figure that reinforces the safety advantages of nonthermal ablation.
No major complications: Importantly, the registry reported no esophageal fistulas or pulmonary vein stenosis, two complications historically associated with thermal ablation methods.
Durable clinical effectiveness: Patients experienced strong maintenance of sinus rhythm at one year, underscoring PFA’s potential to reduce AFib recurrence over time.
These results build on 30-day data shared at HRS 2024, which had already shown promising short-term safety and efficacy. The one-year data confirm that Farapulse delivers sustained outcomes across diverse patient populations and ablation strategies, advancing confidence in PFA as a long-term solution.
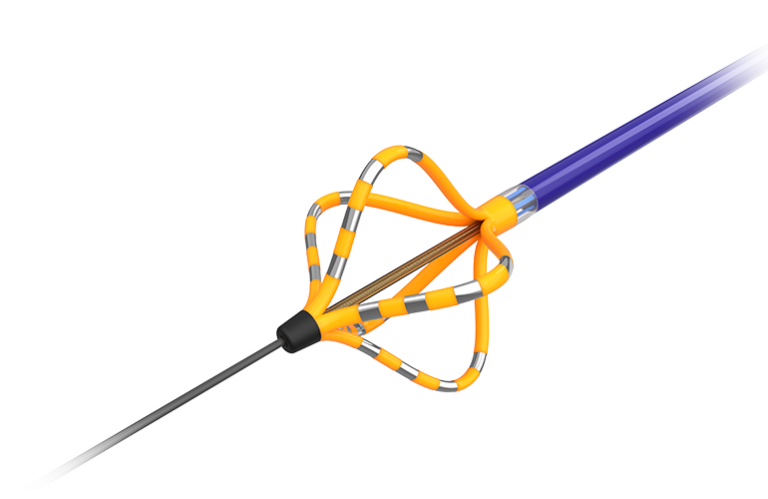
Watchman FLX: new insights from the OPTION trial
Boston Scientific also presented a sub-analysis of the OPTION trial, focused on the next-generation Watchman FLXdevice. Designed with polymer coating, enhanced visualization markers, and a broader size matrix, the FLX system offers physicians greater versatility to treat a wider range of patients with non-valvular AFib.
The sub-analysis reaffirmed that Watchman FLX delivers consistent thromboembolic protection while reducing bleeding risk compared to oral anticoagulation. Notably, the benefit extended to both high-risk and low-risk patient groups, underscoring the device’s broad applicability.
Key takeaways included:
Stroke protection maintained: Watchman FLX continued to demonstrate non-inferiority to oral anticoagulation in preventing thromboembolic events.
Significantly reduced bleeding: Patients experienced fewer major bleeding events, addressing one of the most serious complications associated with long-term anticoagulant use.
Consistency across risk profiles: Outcomes remained favorable regardless of stroke risk, suggesting the device could play a role across a wide spectrum of AFib patients.
Boston Scientific said the findings support the device’s role as a safe and effective alternative to long-term anticoagulation, a message likely to resonate strongly with both physicians and payors.
Strategic positioning in AFib care
By showcasing strong outcomes for both Farapulse and Watchman FLX, Boston Scientific is reinforcing its dominance in structural and electrophysiological solutions for AFib. With AFib prevalence projected to surpass 12 million patients in the U.S. by 2030, demand for both effective ablation and stroke-prevention therapies will continue to climb.
Boston Scientific’s approach is particularly notable because it spans the entire AFib care continuum:
Farapulse for rhythm control, leveraging PFA’s safety and speed advantages over traditional ablation.
Watchman FLX for stroke prevention, offering patients an alternative to lifelong anticoagulants.
The combination positions Boston Scientific as one of the only MedTech leaders capable of addressing AFib from multiple angles, giving it a strategic edge against competitors like Medtronic (with its Affera PFA system) and Abbott(with its Amplatzer Amulet LAAC device).
Competitive positioning: Boston Scientific’s dual play in PFA and LAAC
Boston Scientific’s strong clinical updates on Farapulse and Watchman FLX highlight not just product performance but also the company’s broader strategy: to dominate the two fastest-growing categories in atrial fibrillation (AFib) care—pulsed field ablation (PFA) for rhythm control and left atrial appendage closure (LAAC) for stroke prevention. In doing so, Boston Scientific positions itself at the intersection of two markets projected to expand significantly as AFib prevalence climbs worldwide.
PFA: challenging Medtronic, J&J, and Kardium
The pulsed field ablation market is one of the most competitive in cardiovascular medicine today. PFA is rapidly gaining traction as the preferred alternative to radiofrequency (RF) and cryoablation, thanks to its safety profile and procedural efficiency.
Boston Scientific’s Farapulse system is a clear leader:
It has CE Mark in Europe, where adoption is accelerating.
It won FDA approval in early 2024 and an expanded approval that summer, giving it a foothold in the U.S. ahead of many competitors.
Clinical data continues to show consistent, durable outcomes across AFib subtypes.
The competitive set includes:
Medtronic (Affera Sphere-9): A combined mapping and ablation platform, acquired in 2022. Medtronic brings scale and distribution power, though its PFA system is still working to match Farapulse’s clinical visibility.
Johnson & Johnson (Varipulse): Leveraging its Biosense Webster mapping dominance, J&J is integrating PFA into its Carto ecosystem. However, regulatory progress lags behind Boston Scientific.
Kardium (Globe system): Recently FDA-approved, Globe integrates mapping and ablation in a single catheter with a 122-electrode array. While innovative, Kardium lacks the commercial infrastructure of Boston Scientific.
Farapulse’s competitive advantage lies in being first to scale with robust real-world evidence. The FARADISE registry data presented at ESC 2025 gives Boston Scientific not only clinical credibility but also a marketing edge in physician adoption.
LAAC: outpacing Abbott with Watchman FLX
In the left atrial appendage closure (LAAC) space, Boston Scientific’s Watchman FLX is the dominant global brand. The OPTION trial sub-analysis presented at ESC 2025 reinforced its dual value proposition:
Stroke protection comparable to oral anticoagulants.
Significant reduction in bleeding risk, regardless of patient risk profile.
The Watchman franchise already enjoys strong market penetration in the U.S. and Europe. Abbott’s Amplatzer Amulet is the primary competitor, offering an alternative LAAC device with a different design philosophy. While Amulet has gained FDA approval, it has not yet matched Watchman FLX’s scale of adoption or depth of supporting evidence.
Boston Scientific continues to build on this lead with incremental innovation—the latest FLX iteration includes polymer coatings, visualization markers, and a broader size matrix to address more patient anatomies. This positions Watchman as a solution that can reach wider populations while maintaining clinical performance.
Strategic advantage: a full AFib portfolio
What sets Boston Scientific apart is its two-pronged portfolio strategy:
Farapulse attacks AFib at its source by eliminating arrhythmogenic tissue with PFA.
Watchman FLX mitigates AFib’s most dangerous consequence—stroke—without reliance on long-term anticoagulants.
No other MedTech competitor currently combines leadership positions in both rhythm control and stroke prevention. Medtronic and J&J are strong in electrophysiology but lack equivalent LAAC offerings. Abbott is competitive in LAAC but trails in PFA development.
This portfolio synergy strengthens Boston Scientific’s value proposition to hospitals and physicians. Centers that adopt Farapulse are more likely to integrate Watchman, and vice versa, creating cross-selling opportunities and deepening institutional relationships.
Market implications
The AFib market is vast and growing. In the U.S. alone, AFib prevalence is expected to exceed 12 million patients by 2030, with global numbers rising sharply due to aging demographics. Within this population:
A large proportion will undergo catheter ablation as first-line or second-line therapy.
Millions will require stroke prevention solutions, especially those unsuitable for anticoagulation.
By anchoring both sides of the AFib care pathway, Boston Scientific is positioning itself as the go-to partner for comprehensive AFib management.
Powered by Froala Editor